
Manage Your Regulatory Responsibilities in a Few Clicks
MediRegs helps pharmacies and pharmaceutical distribution companies comply with their Regulatory Responsibilities (Bona Fides) without the headaches.
“MediRegs is a great tool which saved us a lot of time when we were acquiring our MHRA licences manually… As a result our regulatory inspection went better than expected.”
Matthew Yung. Communications Manager. G D Cooper & Co. Ltd.
Don’t Get Caught Out
MediRegs authenticates your supply chain and verifies if your trading or distribution partners are authorised or entitled to trade or supply specific pharmaceutical products.
Reduce Your Risk of Fines
MediRegs supports the Guidelines on Good Distribution Practice (GDP) by providing an audit trail for regulatory inspections to keep you compliant and reduce the risk of fines or prosecution from the licensing authority.
Know Before It’s Too Late
MediRegs will automatically alert you if any customers’ or suppliers’ licenses are suspended or revoked. You can also download current or historical reports for their trading status.
Save Time and Money
MediRegs transforms a time intensive, repetitive and manual process into one that is simple, fast and efficient. It validates WDA and GPhC data – saving you hours of checking your company records after every license update..
Watch the full demo:
MediRegs helps you to:
-
Know if any supplier or customer has had their pharmaceutical authorisations changed
-
Check multiple sites and every license type for any organisation
-
Keep track of your License Holder information
-
See the changes approved by the Licensing Authority (MHRA, GPhC or PSNI) during a specified time period
-
Manage your business from anywhere, with a fully mobile responsive application developed for desktop, iOS & Android
Reduce the Possibility of Human Error:
-
Automate your current time-consuming manual processes to reduce the chance of human error.
-
Utilise staff resources more efficiently by quickly verifying your supply chain in just a few clicks.
-
Grant full, or ‘read-only’ access to any of your GDP or sales team staff. Record the audit trail to show which team members updated what and when.
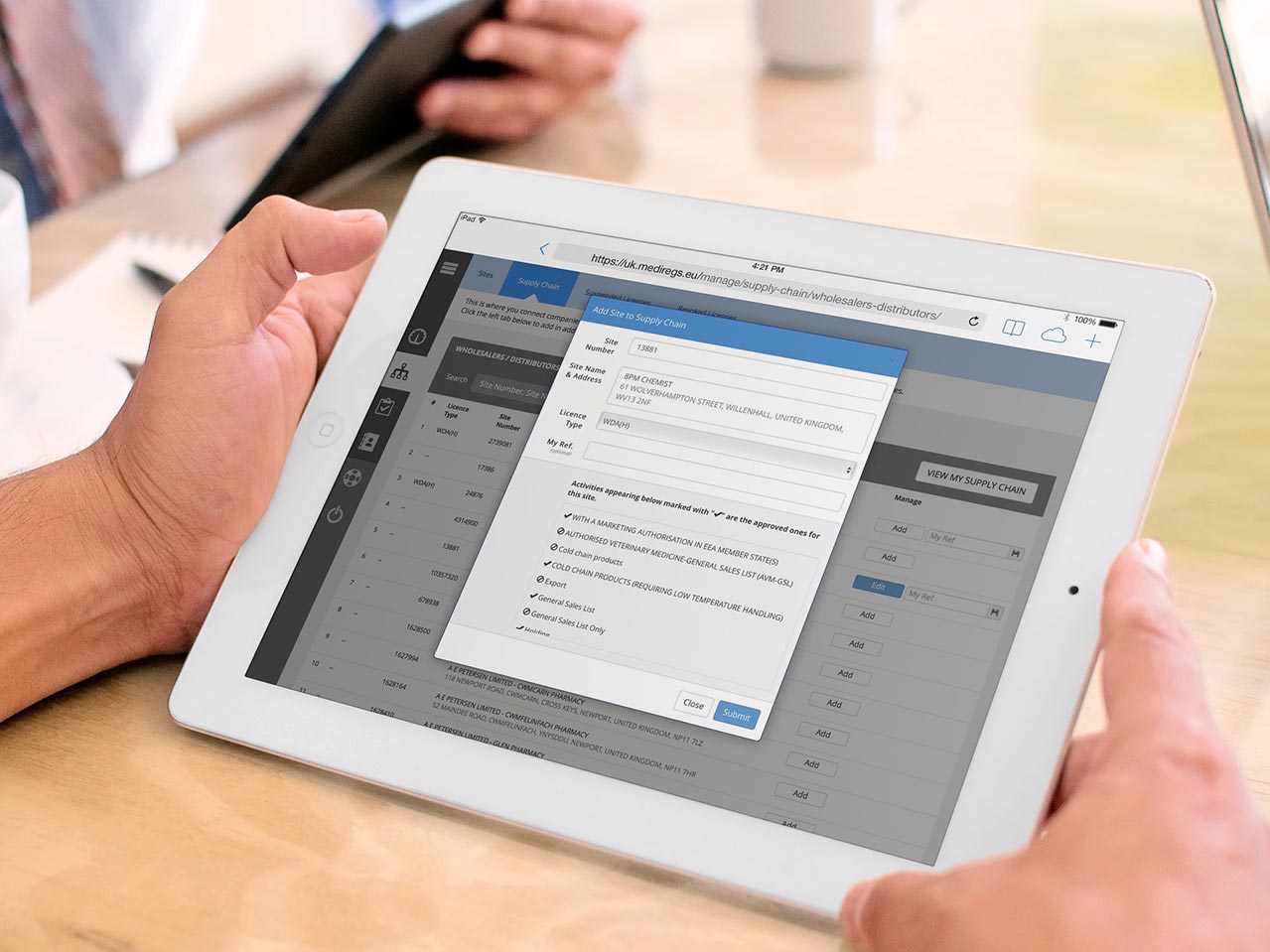
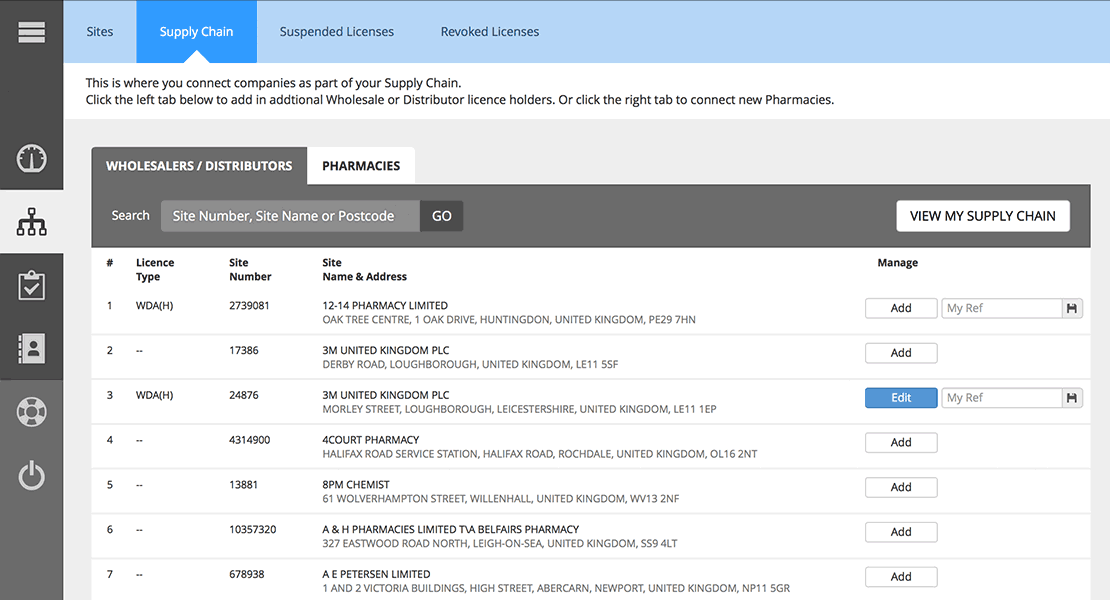
Easily Manage Your Supply Chain Regulatory Requirements
Know instantly if any supplier or customer has their pharmaceutical authorisations changed by the MHRA or GPhC. Reduce the risk of breaching your regulatory responsibilities by validating which organisations have the appropriate authority to trade in pharmaceuticals.
Verify your customers’ and suppliers’ authorisations – both current and historical. Check multiple sites and every license type for any organisation – not just their head office.
See the changes approved by the Licensing Authority during a specified time period for Wholesale Dealers and Active Substance Manufacturers, as well as importers and Distributors, and the types of product(s) or activities that are handled at the sites.
Keep track of your License Holder information and the type of products or activities that are approved by the regulator for each of your sites. You can also report where your licenses are cited on a Wholesale Dealers License and the type(s) or products or activities that are handled under that License.
Automate your current manual processes
- Utilise staff resources more efficiently
- Verify your customers’ and suppliers’ authorisations
- Reduce the risk of breaching your regulatory responsibilities
Get started for Free
Free Onboarding: We set up the account and configure your connections.
Want to know more? Call us now on 020 7871 0900 or product.info@mediregs.eu for more details
STARTER
£FREELimited to 10 connections
Small WDA holders
- Manage Your Supply Chain
- Manual Verification Reports
- Single User
REGULAR
£99Limited to 50 connections
Distributors / Wholesalers
- Manage Your Supply Chain
- Verification Reports
- Multiple Users
- Automatic License Alerts
- GPhC Data
- Sub-User Management
- + User Activity Tracking
PROFESSIONAL
£179Unlimited connections
Large Distributors / Wholesalers & Teams
- Manage Your Supply Chain
- Verification Reports
- Multiple Users
- Automatic License Alerts
- GPhC Data
- Sub-User Management
- + User Activity Tracking
- Historical Report Archive
Contact us for Enterprise options: Multiple WDA Licences & Custom Domain Name.
Now Including individual Pharmacy Premises data from the GPhC and PSNI
A secure solution developed by a company with a track record of solving problems in the Health sector
Common questions about MediRegs
Contact product.info@mediregs.eu if you have any questions and we’ll do our best to answer.
-
WHAT IS THE MINIMUM CONTRACT TERM?
There is no minimum term contract. The Service begins as soon as your initial payment is processed. You’ll be charged the rate stated at the time of purchase, every month, until you cancel. You can cancel at anytime.
-
WILL THE SITE WORK ON A SMARTPHONE OR TABLET?
Yes. The site is developed using the Bootstrap framework which is the world’s most popular HTML, CSS, and JavaScript framework for developing responsive, mobile-first web sites. This means you can use the site perfectly on a mobile device such as an iPhone or iPad, as well as other Android devices.
-
CAN I UPGRADE / DOWNGRADE MY ACCOUNT?
Yes, you can either upgrade or downgrade your account at any time. Please note, downgrading your Service will cause the loss of the extra features from the higher level account. E.g. If you downgrade from the 5 user account to the single user account, any additional users that you invited will be unable to log in whilst you remain on the lower account tariff.
-
DO THE MONTHLY PRICES INCLUDE VAT?
The prices stated on the home page are exclusive of VAT, which will be applied at the standard UK rate of 20%. Inclusive prices (as March 2021) are:
Starter: FREE
Regular: £79 pcm
Professional: £119 pcm -
HOW MUCH DOES BESPOKE ONBOARDING COST?
We recognise that many companies have their EUFMD data stored in legacy formats across multiple locations. Sometimes this might be internal databases, excel documents, or even hardcopy spreadsheets updated by hand, then duplicated and distributed. Getting all this complex information into a new format can be a lengthy and time-consuming process – especially if there are thousands of records.
We offer a bespoke onboarding service that can take all your EUFMD data and upload it into your MediRegs account. The cost for this service depend on the amount of data, the complexity and the formats involved. Please contact us for a quote.
-
WHERE IS THE DATA STORED?
The site is hosted in the Equinix Datacentre in Slough. The data centre facilities are totally secure, fully air conditioned with redundant power supplies and are fully supported 24/7 by an on-site Network Operations Team. Full address for any GDPR enquiries is Equinix, 8 Buckingham Ave, Slough SL1 4AX UK
-
IS MY DATA SAFE?
MediRegs is developed by South˚. We are registered with the Information Commissioner. We have notified the ICO and our data protection number is Z2907170. All data connections are across SSL using a 256bit key from Comodo with an AES 256bit encryption algorithm. We backup to a secure offsite location using R1Soft, which offers continuous data protection only backing up what has changed – but results in full restore points at any one time. Backups are taken daily at 9AM and 10 of these are retained, with 8 weekly archives – so we have backups every day for 10 days ago and 8 weeks worth of backups available.






